Dr. Peter LeWitt's Laboratory

Professor of Neurology
Sastry Foundation Endowed Chair
Director of Movement Disorder
Fellowship Program WSU
Clinic Appointments: (313) 745-4275
Publications
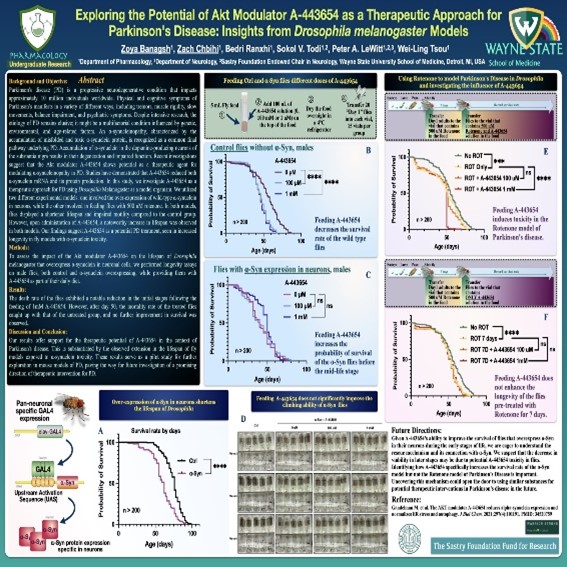
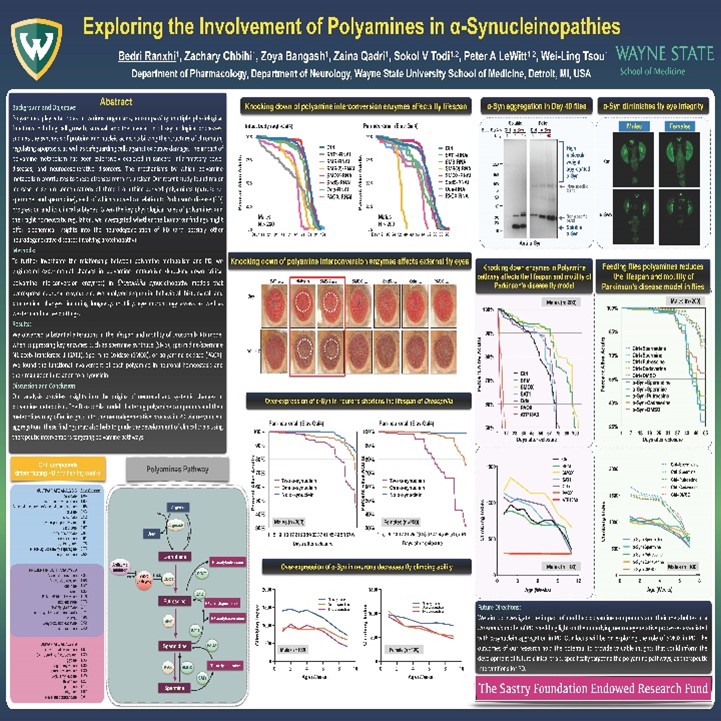
Dr. LeWitt’s research efforts include studies of biomarkers for Parkinson disease and other neurodegenerative disorders. Using biospecimen repositories created from clinical trials and other resources (such as samples paired with autopsy-proven pathological diagnosis), these studies have identified unique combinations of biochemicals that may enhance the sensitivity and specificity of diagnosis. Another recent study has demonstrated that a class of biochemicals derived from L-ornithine (called polyamines) offer markers of PD progression when measured in blood specimens. Polyamine metabolism has been under investigation in a model of Parkinson disease created in Drosophila (fruit fly), in collaboration with Department of Pharmacology researchers Wei-Ling Tsou Ph.D., Sokol Todi, Ph.D., and Bedri Ranxhi. These biomarker findings are aiming to understand the mechanism of progressive changes in L-ornithine-derived polyamine in the neurodegenerative process of Parkinson disease.
 |  |
Farbman ES, Waters CH, LeWitt PA, Rudsińska M, Klingler M, Lee A, Qian J, Oh C, Hauser RA. A 12-month, dose-level blinded safety and efficacy study of levodopa inhalation powder (CVT-301, Inbrija) in patients with Parkinson's disease. Parkinsonism Relat Disord 81:144-150, 2020
Hauser RA, LeWitt PA, Comella CL. On demand therapy for Parkinson’s disease patients? Opportunities and choices. Postgrad Med 133:721-727, 2021
Poewe W, Stocchi F, Arkadir D, Ebersbach G, Ellenbogen AL, Giladi N, Isaacson SH, Kieburtz K, LeWitt P, Olanow CW, Simuni T, Thomas A, Zlotogorski A, Adar L, Case R, Oren S, Orenbach SF, Rosenfeld O, Sasson N, Yardeni T, Espay AJ for the BeyoND Study Group. Subcutaneous levodopa infusion for Parkinson’s disease: one-year data from the open-label BeyoND study group. Mov Disord 36: 2687-2692, 2021
Hauser RA, Hattori N, Fernandez H, Isaacson SH, Mochizuki H, Rascol O, Stocchi F, Li J, Mori A, Nakajima Y, Ristuccia R, LeWitt P. . Efficacy of istradefylline, an adenosine A2A receptor antagonist, as adjunctive therapy to levodopa in Parkinson’s disease: a pooled analysis of 8 phase 2b/3 trials. J Parkinson Dis 11:1663-1675, 2021
Parkinson Study Group SURE-PD Investigators (including PA LeWitt). Effect of urate-elevating inosine on early Parkinson’s disease progression: The SURE-PD3 randomized clinical trial. JAMA 326: 926-939, 2021
Silbergleit AK, Schultz L, David K, LeWitt PA, Sidiropolous C. Self-Perception of voice and swallowing handicap in Parkinson disease. J Parkinson Dis 11:2027-2034, 2021
Jennings D, Huntwork-Rodriguez S, Henry AG, Sasaki JC, Meisner R, Diaz D, Solanoy H, Wang X, Negrou E, Bondar VV, Chosh R, Maloney MT, Propson NE, Zhu Y, Maciuca R, Harris L, Kay A, LeWitt P, King A,3 Kern D, Ellenbogen A, Goodman I, Siderowf A, Aldred J, Omidvar O, Massoud S, Arguello A, Estrada AA,1 de Vicente J, Sweeney JK, Astarita G, Borin MT, Wong BK, Wong H, Nguyen H, Scearce-Levie K, Ho C, Troyer MD. Safety, tolerability, and pharmacodynamics of LRRK2 inhibitor DNL201: from preclinical studies to Parkinson’s clinical trials. Sci Transl Med Jun 8;14(648):eabj2658. doi: 10.1126/scitranslmed.abj2658.
Martinez-Nunez AE, Sidiropoulos C, Schwalb J, Air E, LeWitt P, Bulica B, Kaminski P, Patel N. Adjuvant medical therapy in cervical dystonia after deep brain stimulation: a retrospective analysis. Front Neurol 2022; https://doi.org/10.3389/fneur.2022.927573
LeWitt PA, Stocchi F, Arkadir D, Caraco Y, Adar L, Perlstein I, Case R, Giladi N. The pharmacokinetics of continuous subcutaneous levodopa/carbidopa infusion: findings from the ND0612 clinical development program. Frontiers Neurol 13:1036068. doi: 10.3389/fneur.2022.1036068. 2022
LeWitt PA, Li J, Wu K-H, Lu M. Diagnostic metabolomic profiling of Parkinson disease biospecimens. Neurobiol Dis Feb;177:105962, 2023. Epub 2022 Dec 20
Hauser RA, LeWitt PA, Waters CH, Grosset DG, Blank B. The clinical development of levodopa inhalation powder. Clin Neuropharmacol 46:66-78, 2023
LeWitt P, Ellenbogen A, Burdick D, Gunzler S, Gil R, Dhall R, Banisadr G, D’Souza R. Improving levodopa delivery: IPX203, a new extended-release carbidopa-levodopa. Clin Parkinsonism Relat Disord 24;8:100197, 2023
Martinez-Nunez AE, LeWitt PA. Drugs to the rescue: comparison of on-demand therapies for OFF symptoms in Parkinson’s disease. J Parkinsons Dis 13:441-451, 2023
Borovečki F, Perković R, Kovacs N, LeWitt PA, Bar MR, Katzenschlager R. Are Parkinson’s disease patients referred too late for device-aided therapies and how can better informed and earlier referrals be encouraged? J Neural Transm (Vienna);130:1405-1409, 2023
LeWitt P, Liang GS, Olanow CW, Kieburtz KD, Jimenez R, Olson K, Klepitskaya O, Loewen G. Opicapone pharmacokinetics and effects on catechol-O-methyltransferase activity and levodopa pharmacokinetics in patients with Parkinson disease receiving carbidopa/levodopa. Clin Neuropharmacol 46:43-50, 2023
LeWitt PA, Stebbins GT, Christensen KV, Riswanto T, Pretorius A, Thomsen M. Buspirone and zolmitriptan combination for dyskinesia: A randomized, controlled, crossover study in patients with Parkinson’s disease. Mov Disord 39:613-618, 2024