Multiple Sclerosis and Neuroimmunology Research
An important part of our clinical research is our image- processing and OCT laboratories; one of the few in the country. Following the tradition established by our late Chair and director, Dr. Khan, our research focuses on the use of novel technology to better understand the demyelinating pathology and promote new therapeutic approaches. Our goal is to achieve neuro repair and neuroprotection, minimize the disabling effect of the disease and contribute to a better quality of life for our patients (neuroimaging/OCT page).
Our laboratory research is very active. In the laboratories of Robert Lisak, MD and Joyce Benjamins, PhD and Paula Dore-Duffy, PhD, pioneering research about human immunology is ongoing. Using animal models and tissue cultures, the investigators study the interactions between different immune mediators and the central nervous system, lymphocyte subsets, and the physiology of blood brain barrier (basic research page).
Our research has been funded by the National Institute of Health, National MS Society, the Sastry Family Foundation and pharmaceutical companies.
Clinical Trials
We participate in a number of clinical trials including:
ADAMAS I
A 3-Arm, Multicenter, Double-Blind, Placebo-Controlled, Randomized Study to Assess the Efficacy and Safety of ADS-5102 Amantadine Extended Release Capsules in Multiple Sclerosis Patients with Walking Impairment (currently enrolling)
ADAMAS II (open label)
A Multicenter, Open-Label Safety and Efficacy Study of ADS-5102 Amantadine Extended Release Capsules in Patients with Multiple Sclerosis and Walking Impairment (currently enrolling)
BIOTIN
Effect of MD1003 in progressive multiple sclerosis: a randomized double blind placebo controlled study. MD1003CT2016-01MS-SPI2
ASCLEPIOS I (COMB157G2301)
Title of Study: A randomized, double-blind, double-dummy, parallel-group study comparing the efficacy and safety of ofatumumab versus teriflunomide in patients with relapsing multiple sclerosis COMB157G2301
OPTIONS
A Multicenter, Randomized, Double Blind, Placebo Controlled Parallel Group, Pilot Study to Assess the Efficacy and Safety of H.P. Acthar® Gel in Subjects with Relapsing-remitting Multiple Sclerosis Protocol Number: MNK14274069 (currently enrolling)
ULTIMATE I study
Phase 3: Ublituximab in MS treatment effects. TG1101-RMS 301
Role: Principal Investigator
CHUGAI
A multicenter, randomized, double- blind, placebo-controlled, phase 3 study to evaluate the efficacy and safety of SA237 as monotherapy in patients with neuromyelitis optica (NMO) and Neuromyelitis Optica spectrum disorder (NMOSD).
CHORDS
An Open-Label Study to Evaluate the Effectiveness and Safety of Ocrelizumab in Patient with Relapsing Remitting Multiple Sclerosis Who Have Had a Suboptimal Response to an Adequate Course of Disease-Modifying Treatment MN30035
ORATORIO
A Phase III, multicenter, randomized, parallel-group, double blinded, placebo controlled study to evaluate the efficacy and safety of ocrelizumab in adults with Primary Progressive Multiple Sclerosis WA25046
MEDIMMUNE
A Double-masked, Placebo-controlled Study with Open-Label Period to Evaluate the Efficacy and Safety of MEDI-551 in Adult Subjects with Neuromyelitis Optica and Neuromyelitis Optica Spectrum Disorders (currently enrolling)
OPERA
A Randomized, Double-Blind, Double-Dummy, Parallel-Group Study To Evaluate The Efficacy And Safety Of Ocrelizumab In Comparison To Interferon Beta-1a (Rebif®) In Patients With Relapsing Multiple Sclerosis. WA21093
TOPAZ
A long-term follow-up study for Multiple Sclerosis patients who have completed the alemtuzumab Extension Study (CAMMS03409)
PARADIGMS
A two year, double- blind, randomized, multicenter, active-controlled study to evaluate the safety and efficacy of fingolimod administered orally once daily versus interferon β-1a i.m. once weekly in pediatric patients with multiple sclerosis
EXPAND
A multicenter, randomized, double-blind, parallel-group, placebo-controlled variable treatment duration study evaluating the efficacy and safety of Siponimod (BAF312) in patients with secondary progressive multiple sclerosis
ASSESS
A 12-month, randomized, rater- and dose-blinded study to compare the efficacy and safety of fingolimod 0.25 mg and 0.5 mg administered orally once daily with glatiramer acetate 20 mg administered subcutaneously once daily in patients with relapsing-remitting multiple sclerosis
CARE-MS I
A Phase 3 Randomized, Rater-Blinded Study Comparing Two Annual Cycles of IntravenousAlemtuzumab to Three-Times Weekly Subcutaneous Interferon Beta-1a (Rebif) in Treatment-Naïve Patients with Relapsing-Remitting Multiple Sclerosis; 2008-2013
CARE-MS II
A Phase 3, Randomized, Rater-and Dose-Blinded Study Comparing Two Annual Cycles of Intravenous Low- and High-Dose Alemtuzumab to Three-Times Weekly Subcutaneous Interferon Beta-1a (Rebif) in Patients with Relapsing-Remitting Multiple Sclerosis Who Have Relapsed on Therapy.
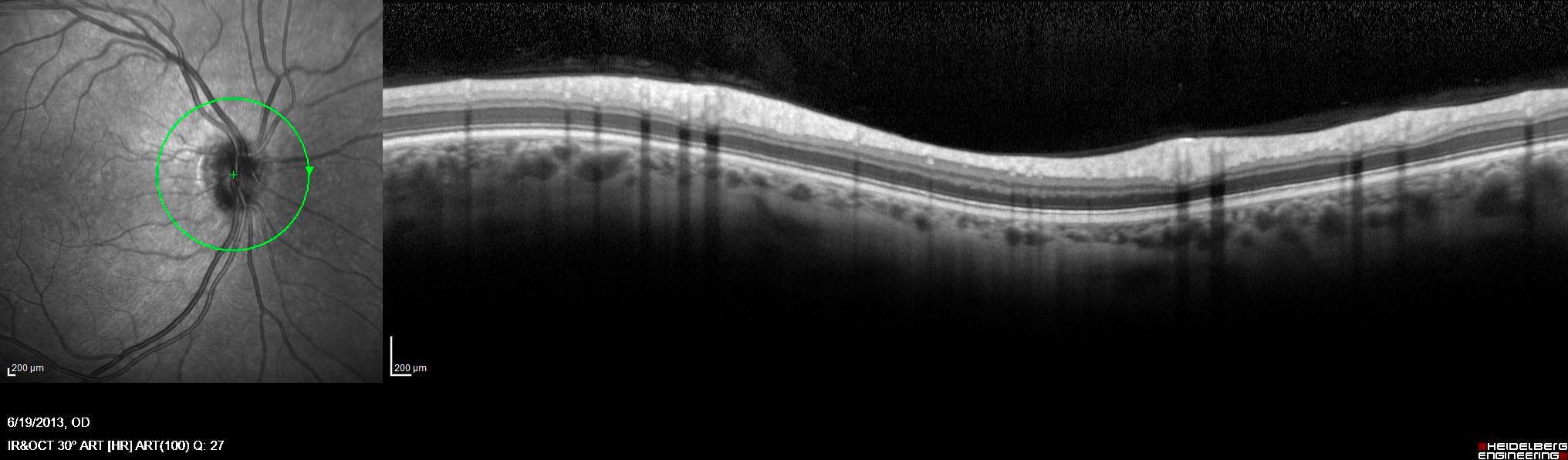
Neuroimaging and OCT
Our neuroimaging and OCT center was established by the late Dr. Omar Khan and is one of the few in the country that applies innovative technology to study the human central nervous system. We are grateful to Sastry Family Foundation for their continuous support.
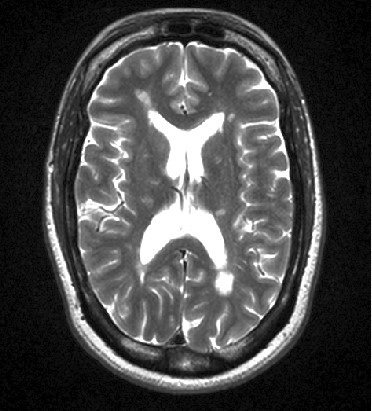
Using a high field (3T) MRI scanner and a 3D Spectralis OCT machine only for research purposes and having our own image processing laboratory, we conduct cutting edge research, studying both the healthy and the diseased brain. Our dedicated team of technicians and coordinators facilitate communication, data collection and help with the execution of the imaging protocols. We have been conducting a number of investigator-initiated trials that improve our understanding on the pathophysiology of demyelinating disorders, the brain repair mechanisms and the effect of disease modifying treatments on MRI and OCT metrics. We are developing new MRI techniques to study disease pathogenesis and recovery potential.
T2W using high filed MRI (3T)
RNFL layer and macular segmentation using a 3D Spectralis OCT